Monoethylene Glycol (MEG) is one of the most common organic compounds, also known as 1 and 2-ethane diol, ethylene alcohol, 1 and 2-dihydroxyethane and hydro-carbonic acid. MEG is the simplest form of Glycols with chemical formula of C2H6O2. Monoethylene Glycol is widely used in production of polyester resins; It is also a major component of antifreeze formulations. MEG is colorless, odorless, and toxic with a sweet taste.
MEG Markets in the World
Asia has a significant share in the production of the global MEG market, as the countries of this region are major producers of fiber & textiles which require Polyester and Polyethylene terephthalate. China, India and Taiwan are the leading producers of MEG among other Asian and Pacific countries.
Iran is also one of the producers that has succeeded in producing MEG 99% high purity. According to an article published in Global Market Insight, Market size of Monoethylene Glycol estimated at USD 25 billion in 2016 and it will grow at a CAGR of more than 6% from 2017 to end of 2024. Demands for MEG is strongly affected by its usage value in textile factories, bottle manufacturers, antifreeze manufacturers and chemical factories.
Supply Monoethylene Glycol from TEAM
TEAM has been actively supplying Monoethylene Glycol to numerous industries and has competitive pricing. Our MEG 99% has been supplied to neighboring countries; references are available upon request. TEAM is also supplier and distributor of other Glycols such as Diethylene Glycol, Triethylene Glycol and Drilling Glycol.
We have a standard quality control system in place and can arrange for international inspection with the help of inspection companies such as SGS.
Monoethylene Glycol Pricing
13 Pricing The price of Monoethylene Glycol may vary depending on its purity and delivery method. Please contact TEAM sales department through our contact numbers, in order to register a purchase order or pre-purchase consultation.
Packaging
Due to the fact that this compound is liquid at normal temperature, MEG chemical compound is supplied in 230 kg drums and/or in IBCs (intermediate bulk containers). Tanks and barrels for storage of this material are made of metal or polyethylene as corrosion resistance is required.
Advantages of Monoethylene Glycol
Exceptional properties of MEG made it one of the main raw materials of various industrial processes. Some of the advantage of MEG is summarized in the following:
The structure of MEG is very similar to water and makes it easy to mix with water. In other words, MEG can be mixed with water without any requirement of special emulsifiers or any other compounds.
MEG has a low freezing point and a high boiling point, which is used in many industrial applications such as coolant and anti-freezing usages
MEG is widely available.
Monoethylene glycol has a lower price compared to other glycols.
MEG has a high thermal stability.
In antifreeze industry, MEG is much more economical than propylene glycol, as a lower percentage of MEG compared to Propylene Glycol is required to reach the same freezing point in water. Furthermore, one of the other advantages of using MEG in antifreeze is its better heat transfer properties than other chemical compounds.
Disadvantages
It is recommended to be extremely careful when working with MEG as it is highly flammable. In case of antifreeze leakage, fire incidents may occur in the vehicles. Reaction of Monoethylene Glycol vapors with air can result in explodes at temperatures above the flash point. Please refer to TEAM MSDS for further details on TEG hazards.
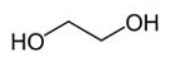
Structural Formula
Monoethylene Glycol (MEG) is an organic compound that contains two hydroxyl groups (OH). The structural formula of MEG is illustrated below: