Understanding Monoethanolamine (MEA): A Comprehensive Overview
Monoethanolamine (MEA), also referred to as ethanolamine or aminoethanol, is a naturally occurring organic chemical compound with a wide range of applications in various industries, particularly within the gas and oil sector. In this article, we'll dive into the world of MEA, exploring its chemistry, uses, production methods, safety, advantages, limitations, and role in critical processes like gas treatment and CO2 capture.
Chemical Composition and Properties of Monoethanolamine (MEA)
MEA Chemical Formula: C2H7NO
Molecular Weight: 61.08 g/mol
Appearance: Colorless, viscous liquid
Odor: Mildly ammoniacal
Solubility: Miscible in water and most organic solvents
pH: Alkaline (approximately 12 in aqueous solution)
Monoethanolamine Structure
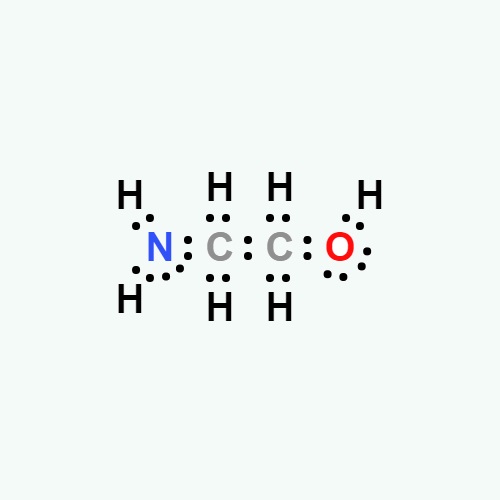
MEA's structural formula, 2-aminoethanol, is represented as HO-CH2-CH2-NH2. This structure is the foundation of MEA's chemical behavior.
Production Methods and Industrial Synthesis of Monoethanolamine (MEA)
The primary method for synthesizing MEA on an industrial scale involves the reaction of ethylene oxide with aqueous ammonia. The process also results in the formation of diethanolamine (DEA) and triethanolamine (TEA). The ratio of these ethanolamines can be adjusted by controlling the reactant proportions.
Applications and Uses of Monoethanolamine (MEA) in Various Industries
Monoethanolamine (MEA) possesses a unique combination of a primary amine group (-NH2) and a hydroxyl group (-OH), making it exceptionally reactive and thus versatile in various industrial applications, including in gas treatment and CO2 capture.
One of the most significant uses of MEA is in amine scrubbing systems. This process removes acid gasses (like hydrogen sulfide (H2S) and carbon dioxide (CO2)) from natural gas and synthesis gas streams.
Exploring the Role of Monoethanolamine (MEA) in Amine Scrubbing Processes
Amine scrubbing with MEA involves two primary steps:
Absorption: Gas streams containing acidic components are brought into contact with an MEA solution. The MEA selectively absorbs CO2 and H2S, removing them from the stream.
Regeneration: The MEA solution, now rich in absorbed gasses, is heated. This triggers the release of CO2 or H2S and regenerates the MEA, allowing it to be reused in the scrubbing cycle.
MEA's ability to absorb CO2 also makes it vital in reducing greenhouse gas emissions and for carbon capture technologies. MEA-based solutions are extensively used in coal-fired power plants and natural gas processing for CO2 removal.
Chemical Reactions and Mechanisms Involving Monoethanolamine (MEA)
MEA's reaction with CO2 lies at the heart of its scrubbing applications as shown in the following equation:
This equation represents the reversible reaction between carbon dioxide (CO2) and Monoethanolamine (MEA). This reaction is the basis for MEA's use in carbon capture. MEA chemically binds to CO2, forming MEA carbamate. The reaction can be reversed with heat, releasing CO2 and regenerating MEA for reuse.
Other Applications:
Beyond its significant role in the gas and oil sector, MEA's unique properties offer valuable applications across other industries. Here are some of the other common areas where Monoethanolamine is widely used:
Detergents and Soaps: MEA acts as an emulsifier and a pH regulator in the production of various detergents and soaps.
Cosmetics and Personal Care: It's a pH-buffering agent in shampoos, lotions, and other personal care products.
Cement Production: MEA functions as a grinding aid in cement production, improving efficiency and reducing energy needs.
Metalworking Fluids: MEA's corrosion-inhibiting properties make it a valuable additive to metalworking fluids, prolonging tool life.
Pharmaceutical Intermediate: Serves as a precursor in the synthesis of pharmaceuticals.
Advantages and Limitations of Monoethanolamine (MEA) in Industrial Processes
While Monoethanolamine offers distinct advantages in industrial processes, understanding its limitations is equally important for optimized and safe implementation.
Advantages:
High Reactivity with CO2 and H2S: Enables efficient gas sweetening.
Regenerability: MEA can be regenerated and reused in gas treatment processes.
Corrosion Inhibition Properties: Protects industrial equipment.
Low Production Cost: Compared to other amines, MEA's relatively low cost contributes to its widespread use.
Versatile Applications: Useful in a range of industries beyond gas and oil.
Limitations:
Toxicity: Requires careful handling and safety protocols.
Corrosive Nature
Degradation: MEA can degrade over time due to high operating temperatures or the presence of contaminants.
Energy Intensive Regeneration: The process to regenerate MEA can be energy-intensive.
Future Perspectives and Research Directions for Monoethanolamine (MEA) Implementation
The future of Monoethanolamine (MEA) holds exciting possibilities. Research is actively exploring ways to enhance its performance, expand its applications in carbon capture, and make its production more environmentally sustainable. Here are key areas of focus:
MEA Blends: Research focuses on combining MEA with other amines for synergistic performance gains and energy savings.
MEA for Post-combustion CO2 Capture: Development of more cost-effective MEA-based processes for large-scale carbon capture.
Green MEA Production: Emphasis on sustainable and renewable production methods.
Environmental Impacts and Safety Considerations of Monoethanolamine (MEA)
Though MEA is biodegradable, it's important to be mindful of its environmental impact. High concentrations can be toxic to aquatic life, emphasizing responsible disposal practices. Moreover, as a corrosive and irritant substance, appropriate personal protective equipment (PPE), including gloves and goggles, is essential during MEA handling.
TEAM Chemicals: Your Trusted Supplier of High-Quality Monoethanolamine
TEAMChem is a leading supplier of high-quality Monoethanolamine (MEA) in bulk quantities for the oil and gas industry, including MEA supplier in Iran.
Our MEA99 meets stringent specifications to ensure optimal performance. Our commitment to excellence is reflected in:
Exceptional MEA99+ Purity: Our MEA consistently meets or exceeds industry standards, ensuring optimal performance in your processes.
Reliable Supply: Our extensive logistics network and robust supply chain guarantee a steady MEA supply to meet your operational needs.
Competitive Pricing: We offer competitive MEA prices without compromising on quality or service.
Technical Expertise: Our team possesses in-depth knowledge of MEA applications and is ready to assist you in optimizing its use.
Partner with TEAMChem for all your Monoethanolamine requirements and experience the difference a dedicated supplier can make.
Abstract and Conclusion
MEA (Monoethanolamine), with the chemical formula
HOCH2CH2NH2 or C2H7NO, is a simple organic ethanolamines family (amino alcohols) with one primary amine and one alcohol group. MEA is therefore able to react with its both functional groups. MEA is an excellent intermediary alkyl amine for reacting with acids in order to produce salts and soaps. More complex family members of ethanolamines are DEA and TEA. MEA is produced from the reaction between ammonia and ethylene oxide.
MEA is a colorless liquid which has a low volatility at room temperature. The freezing point of MEA can be reduced by dilution with water. It presents an ammoniac odor. Mono Ethanol Amine absorbs water molecules and carbon dioxide from the air and is miscible with water and alcohols in any proportion.
Nowadays, the demand for MEA is increasing as many industries such as wood preservation, agriculture industry and oil and gas industries are using MEA in their products and processes.
Advantages
Some advantages of MEA are summarized below:
Having favorable physical and chemical properties
Biodegradable
Negligible toxic properties for aquatic organisms
High reaction rate with carbon dioxide
High heat absorption
Disadvantages
It is recommended to be extremely careful when working with MEA, as it is hazardous to humans and the environment. MEA can lead to hazardous effects such as eyes and mouth injury. Please refer to TEAM MSDS for further details on MEA hazards.
Packaging
Due to the fact that this compound is liquid at normal temperature and has corrosive properties, MEA chemical compound is supplied in 230 kg PE drums and/or in IBCs (intermediate bulk containers) in order to prevent corrosion challenges. Tanks and barrels for storage of this material can be selected from corrosion-resistant materials if required.
Market Overview
Referenced to ‘’coherentmarketinsights.com’’ website, the estimation of worldwide market of MEA is anticipated to witness potential picks up within the future and enroll critical CAGR of 5.2% over the period of (2019 – 2027). Worldwide utilization of ethanolamines will proceed to develop at a normal yearly rate of 2.4% in 2021–2026, which is driven by the creating markets in Asia, particularly China. Based on location, MEA market is portioned into North America, Latin America, Europe, Asia Pacific, and Center East. The market in Europe is anticipated to witness noteworthy development, owing to growing personal care industry within the locale. In addition, Europe is anticipated towitness impressive development credited to expanding request from development and pharmaceutical applications.
Purchasing From TEAM
TEAM as an Iranian supplier of Monoethanolamine has been actively supplying this product to numerous industries and has competitive pricing. Our MEA has been supplied to neighboring countries; references are available upon request. We have a standard quality control system in place and can arrange for international inspection with the help of inspection companies such as SGS.
Pricing
The price of Monoethanolamine (MEA) may vary depending on its purity and delivery method. Please contact TEAM sales department through our contact numbers, in order to register a purchase order or pre-purchase consultation.